ตำรายาของประเทศไทย
Thai Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health2,4-Dinitrofluorobenzene (1-Fluoro-2,4-dinitrobenzene) C6H3FN2O4 = 186.10
Use general reagent grade of commerce.
DESCRIPTION Pale yellow, vesicatory crystals, lumps or liquid with a lachrymatory vapour.
MELTING TEMPERATURE About 29º (Appendix 4.3).
WEIGHT PER MILLILITRE About 1.48 g (Appendix 4.9).
REFRACTIVE INDEX About 1.569, at 20º (Appendix 4.7).
Diphenylamine C12H11N = 169.23
Use analytical reagent grade of commerce.
DESCRIPTION White crystals; odour, characteristic.
MELTING TEMPERATURE About 55º (Appendix 4.3).
Store protected from light.
Diphenylbenzidine (N,N’-Diphenylbenzidine) C24H20N2 = 336.44
Use general reagent grade of commerce.
DESCRIPTION White or faintly grey-coloured, crystalline powder.
SOLUBILITY Insoluble in water; slightly soluble in acetone and in ethanol.
MELTING RANGE 246º to 250º (Appendix 4.3).
Store protected from light.
Dipotassium Edetate (Dipotassium Dihydrogen Ethylenediaminetetra-acetate) C10H14N2K2O8.2H2O = 404.46
Use general reagent grade of commerce.
Dipotassium Hydrogenphosphate (Potassium Phosphate, Dibasic) K2HPO4 = 174.18
Use general reagent grade of commerce.
DESCRIPTION White, crystalline powder.
SOLUBILITY Soluble in water.
Disodium Edetate C10H14N2Na2O8.2H2O = 372.24
Use analytical reagent grade of commerce.
Disodium Hydrogenphosphate Na2HPO4.12H2O = 358.14
Use analytical reagent grade of commerce.
DESCRIPTION Translucent crystals or granules.
SOLUBILITY Freely soluble in water; practically insoluble in ethanol.
Disodium Hydrogenphosphate, Anhydrous Na2HPO4 = 141.96
Use analytical reagent grade of commerce.
Disodium Hydrogenphosphate Dihydrate (Disodium Hydrogen Orthophosphate Dihydrate) Na2HPO4.2H2O = 178.00
Use analytical reagent grade of commerce.
Store in tightly closed containers.
Dithiothreitol (Cleland’s Reagent) C4H10O2S2 = 154.24
Use general reagent grade of commerce.
DESCRIPTION Slightly hygroscopic needles.
SOLUBILITY Freely soluble in water, in acetone, in ethanol, in ether, and in ethyl acetate.
MELTING RANGE Between 42º and 44º (Appendix 4.3).
Store in tightly closed containers.
Dithizone (Phenylazothioformic Acid 2- Phenylhydrazide; Diphenylthiocarbazone) C13H12N4S = 256.32
Edetic Acid (Ethylenediaminetetra-acetic Acid) C10H16N2O8 = 292.25
Use general reagent grade of commerce.
DESCRIPTION White, crystalline powder.
SOLUBILITY Very slightly soluble in water; soluble in solutions of alkali hydroxides.
MELTING TEMPERATURE Above 220º, with decomposition (Appendix 4.3).
Eosin Y (Acid Red 87; Eosin Yellowish Y) C20H6Br4Na2O5 = 691.86
Use general reagent grade of commerce.
DESCRIPTION Red to brownish red pieces or powder; dissolves in water to yield yellow purplish red solution with a greenish yellow fluorescence.
SOLUBILITY Soluble in water and in ethanol.
Ethanol C2H6O = 46.07 Use Ethanol (95 Per Cent) (see under “Reagents”).
Ethanol (95 Per Cent)
A mixture of ethanol and water. Contains not less than 92.3 per cent w/w and not more than 93.8 per cent w/w, corresponding to not less than 94.9 per cent v/v and not more than 96.0 per cent v/v, at 15.56°, of C2H6O.
DESCRIPTION Colourless, clear, mobile and volatile liquid; odour, characteristic and spirituous. Flammable, burning with a blue smokeless flame. Boils at about 78°.
SOLUBILITY Miscible with water, with chloroform and with ether.
IDENTIFICATION
A. Mix 5 drops in a small beaker with 1 mL of potassium permanganate TS and 5 drops of dilute sulfuric acid and cover the beaker immediately with a filter paper moistened with a solution recently prepared by dissolving 100 mg of sodium nitroferricyanide and 500 mg of piperazine hydrate in 5 mL of water: an intense blue colour is produced on the filter paper, the colour becoming paler after a few minutes.
B. To 5 mL of a 0.5 per cent v/v solution, add 1 mL of 0.1 M sodium hydroxide; then slowly add 2 mL of iodine TS: the odour of iodoform develops and a yellow precipitate is produced.
ACIDITY OR ALKALINITY To 20 mL add 5 drops of phenolphthalein TS: the solution remains colourless and requires not more than 0.20 mL of 0.10 M sodium hydroxide to produce a pink colour.
CLARITY OF SOLUTION Dilute 5 mL to 100 mL with water in a glass cylinder: the solution remains clear when examined against a black background.
ALDEHYDES AND KETONES Heat 100 mL of hydroxylamine TS in a loosely stoppered flask on a water-bath for 30 minutes, cool, and, if necessary, add sufficient 0.050 M sodium hydroxide to restore the green colour. To 50 mL of this solution add 25 mL of the sample and heat on a water-bath for 10 minutes in a loosely stoppered flask. Cool, transfer to a Nessler cylinder, and titrate with 0.050 M sodium hydroxide until the colour matches that of the remainder of the hydroxylamine solution contained in a similar cylinder, both solutions being viewed down the axis of the cylinder. Not more than 0.90 mL or 0.050 M sodium hydroxide is required.
OXIDIZABLE SUBSTANCES To 20 mL add 1 mL of 0.002 M potassium permanganate. Allow the solution to stand at 20° for 10 minutes protected from light: the colour is not completely discharged.
NON-VOLATILE MATTER A 100-mL sample, when evaporated and dried at 100° to 105° to constant weight, leaves not more than 2.5 mg of residue.
SPECIFIC GRAVITY 0.805 to 0.821, at 25° (Appendix 4.9), using this result to ascertain the percentage of C2H6O contained in the liquid examined by reference to the Alcoholometric Table.
VOLATILE IMPURITIES Carry out the test as described in the “Gas Chromatography” (Appendix 3.4).
Reference solution (a) Dilute 100 mL of anhydrous methanol to 50.0 mL with the test substance. Dilute 5.0 mL of the solution to 50.0 mL with the test substance.
Reference solution (b) Dilute 50 mL of anhydrous methanol and 50 mL of acetaldehyde to 50.0 mL with the test substance. Dilute 100 mL of the solution to 10.0 mL with the test substance.
Reference solution (c) Dilute 150 mL of acetal to 50.0 mL with the test substance. Dilute 100 mL of the solution to 10.0 mL with the test substance.
Reference solution (d) Dilute 100 mL of benzene to 100.0 mL with the test substance. Dilute 100 mL of the solution to 50.0 mL with the test substance.
Test solution (a) The test substance.
Test solution (b) Add 150 mL of 4-methyl-2-pentanol to 500.0 mL of the test substance.
Chromatographic system A gas chromatograph equipped with (a) a glass (fused silica) column (30 m × 0.32 mm) packed with porous poly[(cyanopropyl)(phenyl)][dimethyl]-siloxane (1.8 mm), maintained
as the following table, (b) a flame ionization detector maintained at 280°, and (c) helium as the carries gas.
| Time (Minutes) | Temperature (º) | |
| Column | 0-12 12-32 32-42 |
40 40 → 240 240 |
| Injection Port | 200 |
System suitability Chromatograph Reference solution (b) and record the peak response as directed for Procedure: the resolution between the first peak (acetaldehyde) and the second peak (methanol) is not less than 1.5.
Procedure Inject separately suitable volumes of each of Reference solution (a), Reference solution (b), Reference solution (c), Reference solution (d), Test solution (a), and Test solution (b).
Limits:
- methanol in the chromatogram obtained from test solution (a): not more than half the area of the corresponding peak in the chromatogram obtained from reference solution (a) (200 ppm v/v),
- acetaldehyde + acetal: maximum 10 ppm v/v, expressed as acetaldehyde.
Calculation Calculate the sum of the contents of acetaldehyde and acetal in parts per million (v/v) using the following expression:
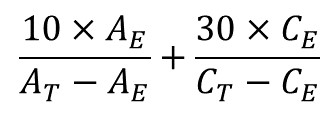
where AE = area of the acetaldehyde peak in the chromatogram obtained from test solution (a),
AT = area of the acetaldehyde peak in the chromatogram obtained from reference solution (b),
CE = area of the acetal peak in the chromatogram obtained from test solution (a), and
CT = area of the acetal peak in the chromatogram obtained from reference solution (c).
- benzene: maximum 2 ppm v/v.
Calculation Calculate the content of benzene inparts per million (v/v) using the following expression:
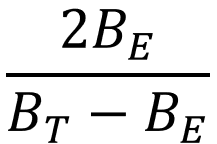
where BE = area of the benzene peak in the chromatogram obtained from the test solution (a), and
BT = area of the benzene peak in the chromatogram obtained from reference solution (d).
If necessary, the identity of benzene can be confirmed using another suitable chromatographic system (stationary phase with a different polarity).
- total of other impurities in the chromatogram obtained from test solution (b): not more than the area of the peak due to 4-methyl-2-pentanol in the chromatogram obtained from test solution (b) (300 ppm v/v),
- disregard limit: 0.03 times the area of the peak corresponding to 4-methyl-2-pentanol in the chromatogram obtained from test solution (b) (9 ppm v/v).
Ethanol, Absolute C2H6O = 46.07
Use analytical reagent grade of commerce containing not less than 99.5 per cent v/v of C2H6O.
DESCRIPTION Colourless, clear, mobile and volatile liquid; odour, characteristic and spirituous. Flammable, burning with a blue, smokeless flame. Hygroscopic.
SOLUBILITY Miscible with water, with chloroform and with ether.
BOILING RANGE 78º to 79º (Appendix 4.5).
RELATIVE DENSITY 0.791 to 0.794 (Appendix 4.9).
Store protected from light at a temperature not exceeding 30º.
Ethanol, Aldehyde-free (Aldehyde-free Alcohol) Mix 1200 ml of ethanol with 5 ml of a 40 per cent w/v solution of silver nitrate and 10 ml of a cooled 50 per cent w/v solution of potassium hydroxide. Shake, allow to stand for a few days and filter. Distil the filtrate immediately before use.
Ethanol, Diluted Prepare by diluting the volumes of ethanol indicated in the following table with water to 1000 ml.
Ethanol, Neutralized To a suitable quantity of ethanol add 2 or 3 drops of phenolphthalein TS and just sufficient 0.02 M or 0.1 M sodium hydroxide to produce a faint pink colour.
Prepare neutralized ethanol just prior to use.
Ether C4H10O = 74.12
|
Caution Ether tends to form explosive peroxides especially when anhydrous |
Use analytical reagent grade of commerce.
DESCRIPTION Clear, colourless, volatile, very mobile liquid; odour, characteristic. Highly flammable; mixtures of its vapour with oxygen, air, or nitrous oxide in certain concentrations are explosive.
SOLUBILITY Soluble in 10 parts of water; miscible with benzene, with chloroform, with dichloromethane, with ethanol, with fixed oils, with petroleum ether, and with volatile oils.
PEROXIDES Transfer 8 ml of potassium iodide and starch TS to a 12-ml ground-glass-stoppered cylinder about 1.5 cm in diameter. Fill completely with the test substance, shake vigorously and allow to stand in the dark for 30 minutes. No colour is produced.
Store protected from light at a temperature not exceeding 15º. The name and concentration of any added stabilizer are stated on the label.
Ether, Peroxide-free (C2H5)2O = 74.12
Shake 1000 ml of ether with 20 ml of a solution of 30 g of iron(II) sulfate in 55 ml of water and 3 ml of sulfuric acid. Continue shaking until a small sample from upper layer no longer produces a blue colour when shaken with an equal volume of a 2 per cent w/v solution of potassium iodide and 1 drop of starch TS. Discard the aqueous layer.
Ethyl Acetate C4H8O2 = 88.11
Use analytical reagent grade of commerce.
DESCRIPTION Colourless liquid; odour, fruity-like.
BOILING RANGE 76º to 78º (Appendix 4.5).
WEIGHT PER MILLILITRE 0.901 to 0.904 g (Appendix 4.9).
Ethylbenzene C8H10 = 106.17
Use general reagent grade of commerce containing not less than 99.5 per cent w/w of C8H10 when determined by gas chromatography.
DESCRIPTION Colourless, flammable liquid.
SOLUBILITY Practically insoluble in water; miscible with the usual organic solvents.
BOILING TEMPERATURE About 135º (Appendix 4.6).
REFRACTIVE INDEX About 1.496, at 20º (Appendix 4.7).
RELATIVE DENSITY About 0.87 (Appendix 4.9).
Ethylene Oxide (Oxirane) C2H4O = 44.05
Use general reagent grade of commerce.
DESCRIPTION Colourless gas.
2-Ethylhexanoic Acid (2-Ethylhexoic Acid) C8H16O2 = 144.21
Use analytical reagent grade of commerce.
DESCRIPTION Colourless liquid.
RELATIVE DENSITY About 0.91, at 20º (Appendix 4.9).
REFRACTIVE INDEX About 1.425 (Appendix 4.7).
Fast Blue B Salt C14H12Cl4N4O2 = 339.18 Stabilized by the addition of zinc chloride.
DESCRIPTION Dark green powder.
Store in tightly closed containers at a temperature between 2° and 8°.
Formaldehyde Solution (Formalin) CH2O = 30.03
Use analytical grade of commerce containing not less than 34.0 per cent w/v and not more than 37.0 per cent w/v of CH2O.
DESCRIPTION Colourless, aqueous solution with a lachrymatory vapour.
WEIGHT PER MILLILITRE About 1.08 g (Appendix 4.9).
Store at a temperature between 15º and 25º.
Formamide CH3NO = 45.04
Use analytical reagent grade of commerce.
DESCRIPTION Colourless, oily liquid.
SOLUBILITY Soluble in water and in ethanol.
WEIGHT PER MILLILITRE About 1.13 g (Appendix 4.9).
Store in tightly closed containers.
Formic Acid CH2O2 = 46.03
Use analytical reagent grade of commerce containing about 90 per cent w/w of CH2O2 and about 23.6 M in strength.
DESCRIPTION Colourless, corrosive liquid; odour, pungent.
WEIGHT PER MILLILITRE About 1.20 g (Appendix 4.9).
Formic Acid, Anhydrous CH2O2 = 46.03
| Caution Show decomposition of this reagent may produce pressure in the bottle. Loosen cap occasionally to vent the gas. |
Use analytical reagent grade formic acid of commerce containing not less than 98.0 per cent w/w of CH2O2.
DESCRIPTION Colourless, corrosive liquid; odour, pungent.
RELATIVE DENSITY About 1.22 (Appendix 4.9).
Fructose (Levulose) C6H12O6 = 180.16
Use general reagent grade of commerce.
DESCRIPTION Colourless crystals or white, crystalline powder; odourless.
SOLUBILITY Freely soluble in water; soluble in ethanol and in methanol.
MELTING TEMPERATURE About 103º, with decomposition (Appendix 4.3).
SPECIFIC ROTATION About –92º at 20º, determined in a 10 per cent w/v solution containing 0.03 per cent v/v of ammonia (Appendix 4.8).
D-Galactose C6H12O6 = 180.16
Use general reagent grade of commerce.
DESCRIPTION White crystalline or finely granulated powder.
SOLUBILITY Soluble in water; very slightly soluble in ethanol.
MELTING TEMPERATURE About 164º, with decomposition (Appendix 4.3).
SPECIFIC ROTATION About +80º at 20º, determined in a 10 per cent w/v solution containing about 0.05 per cent v/v of ammonia (Appendix 4.8).
Gelatin Use general reagent grade of commerce.
DESCRIPTION Colourless or slightly yellow, transparent, brittle, tasteless sheets, flakes, or powder; odourless.
SOLUBILITY Soluble in hot water, acetic acid and glycerol; insoluble in organic solvents.
Glutaraldehyde C5H8O2 = 100.12
Use general reagent grade of commerce.
DESCRIPTION Oily liquid.
SOLUBILITY Freely soluble in water, in benzene, in ethanol, and in ether.
BOILING TEMPERATURE About 188º (Appendix 4.6).
REFRACTIVE INDEX About 1.434 (Appendix 4.7).
Glycerol (Glycerin) C3H8O3 = 92.09
Use analytical reagent grade of commerce.
DESCRIPTION Colourless, viscous liquid.
WEIGHT PER MILLILITRE About 1.26 g (Appendix 4.9).
Glycerol (85 Per Cent) Glycerol containing 12.0 to 16.0 per cent w/w of water.
WEIGHT PER MILLILITRE 1.22 to 1.24 g (Appendix 4.9).
Glyoxal Bis(2-hydroxyanil) C14H12N2O = 240.26
Use general reagent grade of commerce.
DESCRIPTION White crystals.
SOLUBILITY Soluble in hot ethanol.
MELTING RANGE 203º to 205º (Appendix 4.3).
Guaiacol (2-Methoxyphenol) C7H8O2 = 124.14
Use analytical reagent grade of commerce.
DESCRIPTION Crystalline mass or colourless or yellowish liquid. Hygroscopic.
SOLUBILITY Slightly soluble in water; very soluble in dichloromethane; freely soluble in ethanol.
MELTING TEMPERATURE About 28º. (Appendix 4.3).
BOILING TEMPERATURE About 205º. (Appendix 4.6).
Store protected from light.
Guanine C5H5N5O = 151.13
DESCRIPTION White powder.
SOLUBILITY Practically insoluble in water, freely soluble in potassium hydroxide solution and in dilute acids; sparingly soluble in ethanol and in ether.
Helium He = 4.00
Use laboratory cylinder grade of commerce containing not less than 99.995 per cent v/v of He.
n-Heptane C7H16 = 100.20
Use general reagent grade of commerce.
DESCRIPTION Clear, colourless, volatile, flammable liquid; odour, characteristic.
SOLUBILITY Practically insoluble in water; soluble in absolute ethanol; miscible with chloroform, with ether and with most fixed and volatile oils.
BOILING RANGE 94.5º to 99.0º (Appendix 4.5).
REFRACTIVE INDEX 1.387 to 1.388, at 20º (Appendix 4.7).
WEIGHT PER MILLILITRE 0.683 to 0.686 g (Appendix 4.9).
Hexadimethrine Bromide (C13H30Br2N2)n
Use general reagent grade of commerce.
DESCRIPTION White to off-white, amorphous powder. Hygroscopic.
SOLUBILITY Soluble in water up to 10 per cent to give a colourless to light yellow solution.
Store in tightly closed containers.
Hexane C6H14 = 86.18
The hexane fraction from petroleum.
DESCRIPTION Colourless, mobile, highly flammable liquid.
BOILING RANGE Not less than 95 per cent distils between 67° and 70° (Appendix 4.5).
WEIGHT PER MILLILITRE 0.670 to 0.677 g (Appendix 4.9).
NON-VOLATILE MATTER When evaporated on a water-bath and dried at 105° to constant weight, leaves not more than 0.01 per cent w/v of residue.
n-hexane C6H14 = 86.18
Use an analytical reagent grade of commerce usually containing not less than 99 per cent of the pure isomer, n-C6H14.
DESCRIPTION Colourless, flammable liquid.
BOILING RANGE Distils completely over a range of 1°between 67.5° and 69.5° (Appendix 4.5).
REFRACTIVE INDEX 1.374 to 1.375 (Appendix 4.7).
WEIGHT PER MILLILITRE 0.658 to 0.659 g (Appendix 4.9).
Holmium Oxide Ho2O3 = 377.86
Use general reagent grade of commerce.
DESCRIPTION Yellowish powder.
SOLUBILITY Insoluble in water.
Hydrazine Sulfate (Hydrazinium Sulfate) H6N2O4S = 130.12
Use analytical reagent grade of commerce.
DESCRIPTION Colourless crystals or white, crystalline powder.
SOLUBILITY Soluble in about 40 parts of water; practically insoluble in ethanol (25 per cent).
Hydriodic Acid HI = 127.91
|
Caution To avoid possible explosions this acid should be distilled only in inert atmosphere. |
Use analytical reagent grade of commerce containing about 55 per cent w/w of HI (about 7.5 M in strength).
DESCRIPTION Almost colourless liquid when freshly prepared, but rapidly becoming yellow or brown owing to the liberation of iodine.
SOLUBILITY Miscible with water and with ethanol.
WEIGHT PER MILLILITRE About 1.7 g (Appendix 4.9).
Hydrochloric Acid HCl = 36.46
Use analytical reagent grade of commerce.
Where no molarity is indicated use analytical reagent grade of commerce with a relative density of about 1.18, containing not less than 35 per cent w/w and not more than 38 per cent w/w of HCl and about 11.5 M in strength.
DESCRIPTION Clear, colourless, fuming liquid; odour, pungent.
SOLUBILITY Miscible with water.
Solutions of molarity x M should be prepared by diluting 85x ml of hydrochloric acid to 1000 ml with water.
Store in a container of polyethylene or other non-reacting material at a temperature not exceeding 30º.
Hydrochloric Acid, Dilute A 10 per cent w/v solution. Prepare by mixing 226 ml of hydrochloric acid with water to produce 1000 ml.
Hydrochloric Acid, Heavy Metal-Free
Use a suitable reagent grade of commerce. Complies with the requirements prescribed for hydrochloric acid with the following maximum contents of heavy metals in ppm: Arsenic 0.005, Cadmium 0.003, Copper 0.003, Iron 0.05, Mercury 0.005, Nickel 0.004, Lead 0.001, and Zinc 0.005.
Hydrochloric Acid, Dilute, Heavy Metal-Free
Use a suitable reagent grade of commerce. Complies with the requirements prescribed for hydrochloric acid with the following maximum contents of heavy metals in ppm: Arsenic 0.005, Cadmium 0.003, Copper 0.003, Iron 0.05, Mercury 0.005, Nickel 0.004, Lead 0.001, and Zinc 0.005.
Hydrochloric Acid, 0.1 M Methanolic Dilute 8.5 ml of hydrochloric acid in sufficient methanol to produce 1000 ml.
Hydrogen Peroxide Solution, Strong H2O2 = 34.01
Use analytical reagent grade of commerce containing about 30 per cent w/v of H2O2.
DESCRIPTION Colourless liquid.
WEIGHT PER MILLILITRE About 1.10 g (Appendix 4.9).
Hydrogen Sulfide H2S = 34.08
Prepared by the action of hydrochloric acid, diluted with an equal volume of water on iron sulfide, the resulting gas is washed by passing it through water.
DESCRIPTION Colourless, poisonous gas; odour, characteristic and unpleasant.
Hydroxylamine Hydrochloride (Hydroxylammonium Chloride) NH2OH.HCl = 69.49
Use analytical reagent grade of commerce.
DESCRIPTION Colourless crystals or white, crystalline powder.
SOLUBILITY Very soluble in water; soluble in ethanol.
5-Hydroxymethylfurfural C6H6O3 = 126.11
Use general reagent grade of commerce. M
ELTING TEMPERATURE About 32º (Appendix 4.3).
8-Hydroxyquinoline (8-Quinolinol) C9H7NO = 145.16
Use analytical reagent grade of commerce.
DESCRIPTION White to yellowish white, crystalline powder.
MELTING TEMPERATURE About 74º (Appendix 4.3).
Hydroxy Naphthol Blue C20H12N2O11S3Na2 = 598.48 (598.48162)
Use general reagent grade of commerce containing 1 per cent w/w of hydroxy naphthol blue deposited on sodium chloride.
DESCRIPTION Small blue crystals.
SOLUBILITY Freely soluble in water.
Imidazole (Glyoxaline) C3H4N2 = 68.08
Use purified grade of commerce.
DESCRIPTION White, crystalline powder.
SOLUBILITY Soluble in water and in ethanol.
MELTING TEMPERATURE About 90º (Appendix 4.6).
Indophenol Blue C18H16N2O = 276.34
DESCRIPTION Dark purple powder.
SOLUBILITY Soluble in ethanol and in toluene, giving dark purple solutions.
MELTING RANGE 168º to 170º (Appendix 4.3).
Iodine I2 = 253.81
Use analytical reagent grade of commerce.
Iodine Monobromide (Iodine Bromide) IBr = 206.81
Use general reagent grade of commerce.
DESCRIPTION Bluish black or brownish black crystals with a lachrymatory vapour.
MELTING TEMPERATURE About 40º (Appendix 4.3).
BOILING TEMPERATURE About 116º (Appendix 4.6).
Store in a cool place, protected from light.
Iodine Monochloride ICl = 162.36 (162.3575)
Use general reagent grade of commerce.
DESCRIPTION Black crystals.
SOLUBILITY Soluble in water, in acetic acid and in ethanol.
BOILING TEMPERATURE About 97.4º (Appendix 4.6).
5-Iodouracil C4H3IN2O2 = 237.98
Use general reagent grade of commerce.
DESCRIPTION White or almost white crystalline powder.
MELTING TEMPERATURE About 276º, with decomposition (Appendix 4.3).
HOMOGENEITY Examine under the conditions prescribed in the test for Related substances in the monograph for Idoxuridine applying to the plate 5 μl of a 0.025 per cent w/v solution. The chromatogram shows only one principal spot.
Iron(II) Sulfate (Ferrous Sulfate) FeCl4· 7H2O = 278.01
Use a general reagent grade of commerce.
DESCRIPTION Bluish green crystals or pale, crystalline powder; odourless. Efflorescent in air. Oxidizes in moist air, becoming brown.
Store in well-closed containers
Iron(III) Chloride (Ferric Chloride) FeCl3.6H2O = 270.30
Use analytical grade of commerce.
DESCRIPTION Yellowish orange or brownish, crystalline masses; deliquescent.
Store in well-closed containers.
Iron(III) Sulfate Fe2(SO4)3.xH2O Use general reagent grade of commerce.
DESCRIPTION White to yellow, hygroscopic powder which decomposes in air.
Store protected from light.
Isopropyl Myristate (Isopropyl Tetradecanoate) C17H34O2 = 270.45
Use general reagent grade of commerce.
For use as a solvent in sterility test procedures, Isopropyl Myristate conforms to the following additional specification:
pH OF WATER EXTRACT Transfer 100 ml to a 250-ml centrifuge bottle, add 10 ml of twice-distilled water, close the bottle with a suitable closure, and shake vigorously for 60 minutes. Centrifuge the mixture at 1800 rpm for 20 minutes, aspirate the upper (isopropyl myristate) layer, and determine the pH of the residual water layer: the pH is not less than 6.5.
Isopropyl Myristate not conforming to the test for pH of Water Extract may be rendered suitable for use in sterility test procedures as follows:
Using a 20-mm × 20-cm glass column, add activated alumina, and tamp down to a height of 15 cm. Pass 500 ml of the isopropyl myristate through the column, using a slight positive pressure to maintain an even flow, and use the eluate collected directly in the sterility test procedure.
Kaolin, Light A purified native hydrated aluminium silicate containing a suitable dispersing agent.
Use general reagent grade of commerce.
DESCRIPTION Light, white powder free from gritty particles, unctuous to the touch.
SOLUBILITY Practically insoluble in water and in mineral acids.
COARSE PARTICLES Transfer 5.0 g to a stoppered cylinder about 35 mm in diameter and about 16 cm in length, add 60 ml of a 1 per cent w/v solution of sodium pyrophosphate, shake thoroughly, and allow to stand for 5 minutes. By means of a pipette, draw off 50 ml from a point about 5 cm below the surface of the liquid. To the liquid remaining add 50 ml of water, shake, allow to stand for 5 minutes, and draw off 50 ml in the same way as before. Repeat the operation until a total of 400 ml of the suspension has been drawn off under the prescribed conditions. Transfer the remainder to an evaporating dish, and evaporate to dryness on a water-bath. The residue, after drying at 105º, weighs not more than 25 mg.
FINE PARTICLES Disperse 5.0 g in 250 ml of water by shaking vigorously for 2 minutes in a stoppered flask, immediately pour into a glass cylinder 5 cm in diameter, and transfer 20 ml by means of a pipette to a glass dish; evaporate to dryness and dry at 105º to constant weight. Allow the remainder of the suspension to stand for 4 hours at 20º and withdraw a second 20-ml portion, using a pipette with its tip exactly 5 cm below the surface and without disturbing the sediment. Transfer the second portion to a glass dish, evaporate to dryness, and dry at 105º to constant weight. The weight of the residue from the second portion is not less than 70 per cent of the weight of the residue from the first portion.
Lactose C12H22O11.H2O = 360.31
Use analytical reagent grade of commerce.
DESCRIPTION White, free-flowing powder.
SOLUBILITY Freely but slowly soluble in water; practically insoluble in ethanol.
SPECIFIC ROTATION About +52.4º at 20º, determined in a 10 per cent w/v solution (Appendix 4.8).
Lanthanum Nitrate La(NO3)3.6H2O = 433.01
Use atomic absorption spectroscopic grade of commerce.
DESCRIPTION Colourless crystals. Deliquescent.
SOLUBILITY Freely soluble in water.
Store in tightly closed containers.
Lead(II) Acetate C4H6O4Pb.3H2O = 379.34
Use analytical reagent grade of commerce.
DESCRIPTION Small, white, transparent, monoclinic prisms or heavy, crystalline masses; odour, acetous. Efflorescent in warm air. Becomes basic when heated.
SOLUBILITY Soluble in 2 parts of water and in 63 parts of ethanol; freely soluble in glycerol.
Lead(II) Nitrate Pb(NO3)2 = 331.21
Use analytical reagent grade of commerce.
DESCRIPTION Colourless or white crystals, or white crystalline powder.
SOLUBILITY Soluble in water, forming a clear, colourless solution.
Lead(IV) Oxide (Lead Dioxide) PbO2 = 239.20
Use analytical reagent grade of commerce.
DESCRIPTION Dark brown powder.
SOLUBILITY Practically insoluble in water; soluble in hydrochloric acid with evolution of chlorine; soluble in hot concentrated alkali hydroxide solutions.
Lithium Hydroxide LiOH.H2O = 41.96
Use analytical reagent grade of commerce.
Store in tightly closed containers.
Magenta, Basic (Basic Fuchsin) C20H19N3.HCl = 337.85
A mixture of rosaniline and pararosaniline hydrochlorides.
DESCRIPTION Dark green powder or greenish glistening crystalline fragments, having a bronze-like lustre; odour, not more than a faint odour.
SOLUBILITY Soluble in water, in amyl alcohol, and in ethanol; insoluble in ether.
SENSITIVITY To 10 ml of a solution (1 in 500) add 10 ml of ammonia TS and 500 mg of zinc powder, and agitate the mixture: the solution becomes colourless. Place a few drops of the decolorized solution on filter paper and nearby, on the same paper, place a few drops of dilute hydrochloric acid: a red colour develops at the zone of contact.
LOSS ON DRYING Not more than 5.0 per cent w/w after drying at 105º to constant weight (Appendix 4.15).
SULFATED ASH Not more than 0.3 per cent w/w (Appendix 5.3).
Store protected from light.
Magnesium Oxide MgO = 40.30
Use general reagent grade of commerce.
DESCRIPTION White, fine powder.
SOLUBILITY Very slightly soluble in water; insoluble in ethanol, soluble in dilute acids with at most slight effervescence.
Magnesium Sulfate (Epsom Salts) MgSO4.7H2O = 246.47
Use analytical reagent grade of commerce.
DESCRIPTION Brilliant, colourless crystals or white, crystalline powder; odourless. It efflorescent in warm, dry air.
SOLUBILITY Soluble in 1.5 parts of water; very soluble in boiling water; practically insoluble in ethanol.
Manganese(IV) Oxide (Manganese Dioxide) MnO2 = 86.94
Use analytical reagent grade of commerce.
DESCRIPTION Black or brownish black powder.
Manganese(II) Sulfate MnSO4.H2O = 169.01
Use analytical reagent grade of commerce.
DESCRIPTION Pale red, slightly efflorescent powder; odourless.
SOLUBILITY Soluble in water; insoluble in ethanol.
Store in tightly closed containers.
D-Mannose (Mannose) C6H12O6 = 180.16
Use general reagent grade of commerce.
DESCRIPTION Colourless crystals or white, crystalline powder.
MELTING TEMPERATURE About 132º with decomposition (Appendix 4.3).
SPECIFIC ROTATION About +13.7º to +14.2º at 20º, determined in a 20 per cent w/v in water containing about 0.05 per cent w/v of NH3 (Appendix 4.8).
2-Mercaptoethanol C2H6OS = 78.13
Use general reagent grade of commerce.
RELATIVE DENSITY About 1.116 (Appendix 4.9).
BOILING TEMPERATURE About 157º (Appendix 4.6).
Mercury(I) Nitrate (Mercurous Nitrate Dihydrate) Hg2(NO3)2.2H2O = 561.22
Use analytical reagent grade of commerce.
DESCRIPTION Colourless crystals; odourless or slight nitric acid odour.
SOLUBILITY Soluble in water.
MELTING TEMPERATURE About 70º (Appendix 4.3).
Mercury(II) Chloride HgCl2 = 271.50
Use analytical reagent grade of commerce.
DESCRIPTION Heavy, colourless or white, crystalline masses, or white, crystalline powder.
SOLUBILITY Soluble in 15 parts of water and in 3 parts of ethanol.
Mercury(II) Iodide HgI2 = 454.40
Use general reagent grade of commerce.
DESCRIPTION Dense, scarlet, crystalline powder.
SOLUBILITY Slightly soluble in water and in chloroform; soluble in an excess of potassium iodide TS; sparingly soluble in acetone, in ethanol and in ether.
Store protected from light.
Mercury(II) Oxide, Yellow (Mercuric Oxide) HgO = 216.59
Use commercial grade.
DESCRIPTION Orange-yellow, amorphous powder.
Store protected from light.
Metalphthalein (Phthalein Purple) C32H32N2O12.xH2O
Use indicator grade of commerce.
DESCRIPTION Creamy white to brown powder.
SENSITIVITY Dissolve 10 mg in 1 ml of 13.5 M ammonia and dilute with water to 100 ml. To 5 ml of the solution add 95 ml of water, 4 ml of 13.5 M ammonia, 50 ml of ethanol and 0.1 ml of 0.10 M barium chloride; the solution is bluish violet. Add 0.15 ml of 0.10 M disodium edetate: the solution becomes colourless.
Methanesulfonic Acid CH4O3S = 96.10
Use general reagent grade of commerce.
DESCRIPTION Colourless, corrosive liquid.
REFRACTIVE INDEX About 1.430 (Appendix 4.7).
WEIGHT PER MILLILITRE About 1.48 g (Appendix 4.9).
Methanol (Methyl Alcohol) CH4O = 32.04
Use analytical reagent grade of commerce.
DESCRIPTION Colourless liquid.
SOLUBILITY Miscible with water, forming a clear colourless liquid.
BOILING RANGE 64º to 65º (Appendix 4.6).
RELATIVE DENSITY 0.791 to 0.793 (Appendix 4.9).
Methanol, Aldehyde-free Methanol which complies with the following additional test.
ALDEHYDES AND KETONES Not more than 0.001 per cent w/v. Dissolve 25 g of iodine in 1000 ml of methanol. Add this solution, with constant stirring, to 400 ml of 1 M sodium hydroxide and add 150 ml of water. Allow to stand for 16 hours, filter and boil under a reflux condenser until the odour of iodoform is no longer detectable. Distil the resulting solution by fractional distillation.
Methanol, Anhydrous Methanol which complies with the following additional test.
WATER Not more than 0.1 per cent w/w (Appendix 4.12).
Methenamine (Hexamine) C6H12N4 = 140.19
Use analytical reagent grade of commerce containing not less than 99.0 per cent w/w of C6H12N4.
DESCRIPTION White, crystalline powder or colourless crystals.
SOLUBILITY Freely soluble in water; soluble in dichloromethane and in ethanol.
Methylcellulose 450
Use general reagent grade of commerce. The nominal viscosity is 450 mPa.s.
DESCRIPTION White, yellowish white or greyish white powder or granules; almost odourless.
Methyl Red Sodium Salt C15H14N3NaO2 = 291.28
Use analytical reagent grade of commerce.
2-Methoxyethanol (Ethylene Glycol Monomethyl Ether) C3H8O2 = 76.10
Use chromatographic reagent grade of commerce.
DESCRIPTION Clear, colourless liquid.
SOLUBILITY Miscible with water; with ethanol and with ether.
BOILING TEMPERATURE About 125º (Appendix 4.6).
REFRACTIVE INDEX About 1.406, at 20º (Appendix 4.7).
RELATIVE DENSITY About 0.93 (Appendix 4.9).
2-Methyl-1-Propanol (Isobutyl Alcohol) C4H10O = 74.12
Use analytical reagent grade of commerce.
DESCRIPTION Colourless liquid; odour, characteristic.
BOILING TEMPERATURE About 107º (Appendix 4.6).
REFRACTIVE INDEX 1.397 to 1.399, at 15º (Appendix 4.7).
RELATIVE DENSITY About 0.80 (Appendix 4.9).
3-Methyl-2-benzothiazolinone Hydrazone Hydrochloride Hydrate C8H9N3S.HCl.H2O = 233.70
MELTING TEMPERATURE About 270º (Appendix 4.3).
SUITABILITY FOR THE DETERMINATION OF ALDEHYDES To 2 ml of aldehyde-free methanol add 60 μl of a 0.1 per cent w/v solution of propionaldehyde in aldehyde-free methanol and 5 ml of a 0.4 per cent w/v solution of the reagent being examined, mix and allow to stand for 30 minutes. Add 25 ml of a 0.2 per cent w/v solution of iron(III) chloride, dilute to 100 ml with acetone and mix. The absorbance of the resulting solution at 660 nm is not less than 0.62 (Appendix 2.2). Use in the reference cell a solution prepared at the same time and in the same manner but without the propionaldehyde solution.
4-Methyl-2-pentanone (Methyl Isobutyl Ketone) C6H12O = 100.16
DESCRIPTION Clear, colourless, stable liquid; odour, characteristic.
SOLUBILITY Slightly soluble in water; miscible with most organic solvents.
WEIGHT PER MILLILITRE 0.799 to 0.802 g (Appendix 4.9).
BOILING TEMPERATURE About 115º (Appendix 4.5).
Methylene Blue C16H18ClN3S.3H2O = 373.90
Use a redox indicator grade of commerce.
DESCRIPTION Dark green crystals or crystalline powder, having a bronze-like lustre.
SOLUBILITY Soluble in 25 parts of water and in 65 parts of ethanol; soluble in chloroform.
N,N’-Methylenebisacrylamide C7H10N2O2 = 154.17
Use general reagent grade of commerce.
DESCRIPTION White, fine powder.
N-Methylformamide C2H5NO = 59.07
Use analytical reagent grade of commerce containing not less than 99 per cent v/v of C2H5NO when determined by gas chromatography.
Mordant Black 11 (Eriochrome Black T; Solochrome Black) C20H12N3NaO7S = 461.38
Use general reagent grade of commerce.
DESCRIPTION Brownish black powder having a faint, metallic sheen.
SOLUBILITY Soluble in hot water, in ethanol and in methanol.
SENSITIVITY To 10 ml of a 0.0005 per cent w/v solution in a mixture of equal volumes of methanol and water, add 0.25 M sodium hydroxide until the pH is 10: the solution is pure blue in colour and free from cloudiness. Add 0.01 mg of magnesium ion (Mg): the colour of the solution changes to red-violet, and with the continued addition of magnesium ion it becomes wine-red.
Mordant Black 11 Mixture Grind 200 mg of mordant black 11 to a fine powder with 20 g of potassium chloride.
Naphthalene C10H8 = 128.17
DESCRIPTION Monoclinic prismatic plates, or white scales or powder. A solution in petroleum ether shows a purple fluorescence under light from a mercury-arc lamp. Sublimes at temperatures above the melting temperature.
SOLUBILITY Insoluble in water; very soluble in ether, and in fixed and volatile oils; freely soluble in carbon disulfide, in chloroform, in olive oil, and in toluene; soluble in ethanol and in methanol.
MELTING RANGE 80º to 81º (Appendix 4.3).
BOILING RANGE 217º to 219º (Appendix 4.5).
Naphthalene Black 12B (Amido Black 10B; Acid Black 1) C22H14N4Na2O9S2 = 588.47
Use general reagent grade of commerce.
DESCRIPTION Dark brown powder.
1,3-Naphthalenediol (Naphthoresorcinol) C10H8O2 = 160.17
Use general reagent grade of commerce.
DESCRIPTION Greyish white to tan crystals or powder.
SOLUBILITY Sparingly soluble in water, in ethanol and in ether; freely soluble in methanol.
MELTING RANGE 122º to 127º (Appendix 4.3).
SOLUBILITY TEST IN METHANOL Dissolve 500 mg in 50 ml of methanol: the solution is clear and complete.
1-Naphthol (α-Naphthol) C10H8O = 144.17
Use analytical reagent grade of commerce.
DESCRIPTION Colourless or slightly pinkish crystals or crystalline powder.
SOLUBILITY Insoluble in water; soluble in ethanol and in ether. A 20 per cent w/v solution in ethanol yields a not more than slightly opalescent, colourless or almost colourless solution, with no pink tint.
MELTING TEMPERATURE About 95º (Appendix 4.3).
Store protected from light.
2-Naphthol (β-Naphthol) C10H8O = 144.17
Use analytical reagent grade of commerce.
DESCRIPTION Crystalline; odour, faint phenolic.
MELTING TEMPERATURE About 122º (Appendix 4.3).
Store protected from light.
N-(1-Naphthyl)ethylenediamine Dihydrochloride C12H14N2.2HCl = 259.18
Use general reagent grade of commerce which may contain methanol of crystallization.
DESCRIPTION White or cream powder.
SOLUBILITY Soluble in water.
MELTING TEMPREATURE Not less than 188º (Appendix 4.3).
Ninhydrin (Indane-1,2,3-trione) C9H4O3.H2O = 178.14
Use analytical reagent grade of commerce.
DESCRIPTION A very pale yellow, crystalline powder.
MELTING TEMPERATURE About 255º (Appendix 4.3).
Store protected from light.
Nitric Acid HNO3 = 63.01
When no molarity is indicated, use analytical reagent grade of commerce containing about 70.0 per cent w/w of HNO3 and about 16 M in strength.
DESCRIPTION Corrosive, fuming liquid.
WEIGHT PER MILLILITRE About 1.42 g (Appendix 4.9).
When solutions of molarity xM are required, they should be prepared by diluting 63x ml of nitric acid with water to 1000 ml.
Store protected from light.
Nitric Acid, Dilute Mix 106 ml of nitric acid with sufficient water to produce 1000 ml (approximately 10 per cent w/w of HNO3).
Nitric Acid, Dilute, Heavy Metal-Free
Use a suitable reagent grade of commerce. Complies with the requirements prescribed for hydrochloric acid with the following maximum contents of heavy metals in ppm: Arsenic 0.005, Cadmium 0.005, Copper 0.001, Iron 0.02, Mercury 0.002, Nickel 0.005, Lead 0.001, and Zinc 0.01.
Nitric Acid, Heavy Metal-Free
Use a suitable reagent grade of commerce. Complies with the requirements prescribed for hydrochloric acid with the following maximum contents of heavy metals in ppm: Arsenic 0.005, Cadmium 0.005, Copper 0.001, Iron 0.02, Mercury 0.002, Nickel 0.005, Lead 0.001, and Zinc 0.01.
Nitrogen N2 = 28.01
Use laboratory cylinder grade of commerce, washed with water and dried.
Nitrogen for Chromatography Nitrogen containing not less than 99.95 per cent v/v of N2.
Nitromethane CH3NO2 = 61.04
|
Caution Nitromethane forms explosive compounds with amines and strong bases. |
Use general reagent grade of commerce.
DESCRIPTION Colourless liquid.
SOLUBILITY Slightly soluble in water; miscible with ethanol and with ether.
BOILING TEMPERATURE About 102º (Appendix 4.6).
REFRACTIVE INDEX 1.381 to 1.383, at 20º (Appendix 4.7).
RELATIVE DENSITY 1.132 to 1.134 (Appendix 4.9).
Octoxynol 10 (Octoxinol 10) C34H62O11 (average) = 647
Use general reagent grade of commerce (Triton X-100 or equivalent is suitable).
DESCRIPTION Clear, pale yellow, viscous liquid.
Store in tightly closed containers.
Olive Oil
Use a general reagent grade of commerce.
FREEZING TEMPERATURE 110° (Appendix 4.4).
REFRACTIVE INDEX 1.4680 (Appendix 4.7).
WEIGHT PER MILLILITRE About 0.910 g (Appendix 4.9).
Oxalic Acid C2H2O4.2H2O = 126.07
Use general reagent grade of commerce.
DESCRIPTION Colourless crystals.
SOLUBILITY Soluble in water and in ethanol.
Oxygen O2 = 32.00
Use general reagent grade of commerce containing not less than 99 per cent v/v of O2.
DESCRIPTION Colourless gas; odourless. One litre at 0º and at a pressure of 101.3 kPa (about 760 Torr) weighs about 1.429 g.
SOLUBILITY One volume dissolves in about 32 volumes of water and in about 7 volumes of ethanol at 20º and at a pressure of 101.3 kPa (about 760 Torr).
Papain
Use a suitable grade.
DESCRIPTION White to light tan, amorphous powder.
SOLUBILITY Soluble in water; the solution being colourless to light yellow and more or less opalescent; practically insoluble in chloroform, in ethanol and in ether.
Pentane (n-Pentane) C5H12 = 72.15
DESCRIPTION Clear, colourless, flammable liquid.
SOLUBILITY Very slightly soluble in water; miscible with ethanol, with ether and with many organic solvents.
BOILING RANGE Not less than 95 per cent distils between 34º and 36º (Appendix 4.5).
WEIGHT PER MILLILITRE About 0.63 g (Appendix 4.9).
Pepsin
A substance containing a proteolytic enzyme of the gastric secretion of animals, diluted, if necessary, by admixture with lactose or sucrose. Use a grade of commerce capable of digesting 2500 times its own weight of coagulated egg albumen.
DESCRIPTION Colourless, or light buff-coloured, amorphous powder, or translucent scales; odour, faintly meaty.
SOLUBILITY Soluble in water, yielding an opalescent solution; insoluble in ethanol and in ether.
Perchloric Acid HClO4 = 100.46
When no molarity is indicated, use analytical reagent grade of commerce containing not less than 70.0 per cent and not more than 73.0 per cent w/w of HClO4 and about 12 M in strength.
DESCRIPTION Corrosive liquid.
WEIGHT PER MILLILITRE About 1.7 g (Appendix 4.9).
Petroleum Ether (Light Petroleum)
|
Caution Petroleum Ether is dangerously flammable. Keep away from flames and store in tightly closed containers in a cool place. |
DESCRIPTION Colourless, very volatile, highly flammable liquid, obtained from petroleum, consisting of a mixture of the lower members of the paraffin series of hydrocarbons supplied in the following fractions:
boiling range, 30º to 40º; weight per ml, about 0.63 g
boiling range, 40º to 60º; weight per ml, about 0.64 g
boiling range, 50º to 70º; weight per ml, about 0.66 g
boiling range, 60º to 80º; weight per ml, about 0.67 g
boiling range, 80º to 100º; weight per ml, about 0.70 g
boiling range, 100º to 120º; weight per ml, about 0.72 g
boiling range, 120º to 160º; weight per ml, about 0.75 g.