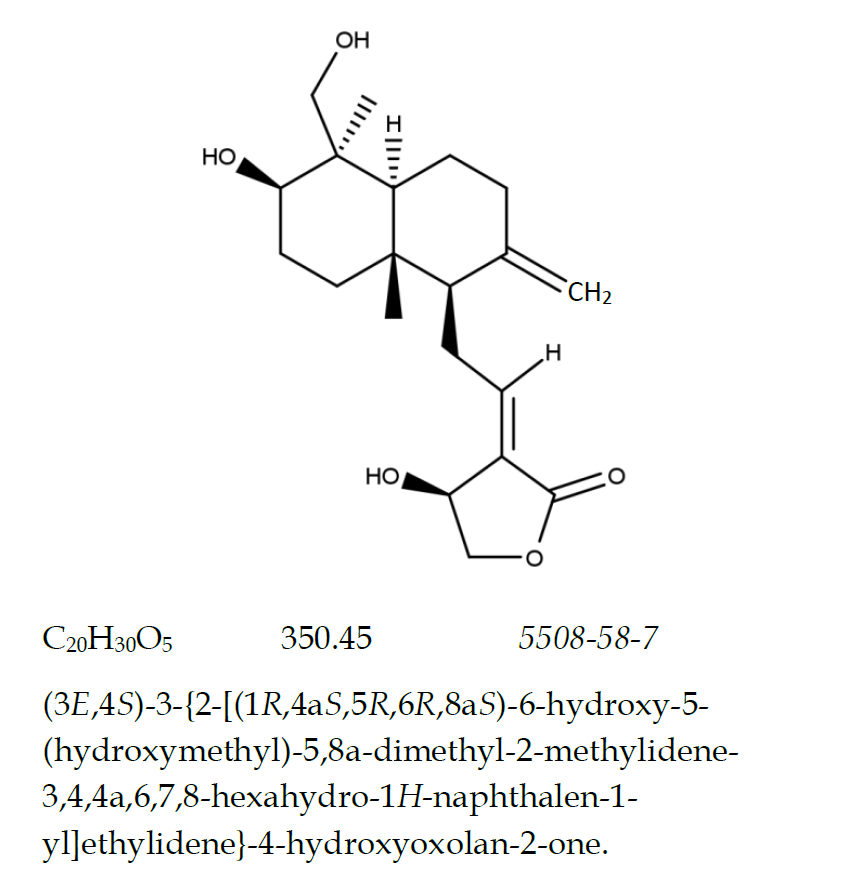
Andrographolide is the diterpene lactone extracted from the dried aerial parts of Andrographis paniculata (Burm. f.) Nees (Family Acanthaceae). It contains not less than 97.0 per cent w/w of C20H30O5.
Description White to off-white powder.
Solubility Insoluble in water; very slightly soluble in chloroform; slightly soluble in ethanol and in methanol.
Other relevant information High storage temperatures, i.e., 70° to 90° result in degradation of andrographolide to other diterpene lactones, particularly 14-deoxy-11,12-didehydroandrog-rapholide, which may decrease blood pressure when consumed in a large amount.
Packaging and storage Andrographolide shall be kept in well-closed containers, protected from light and stored at a temperature of 2° to 8°.
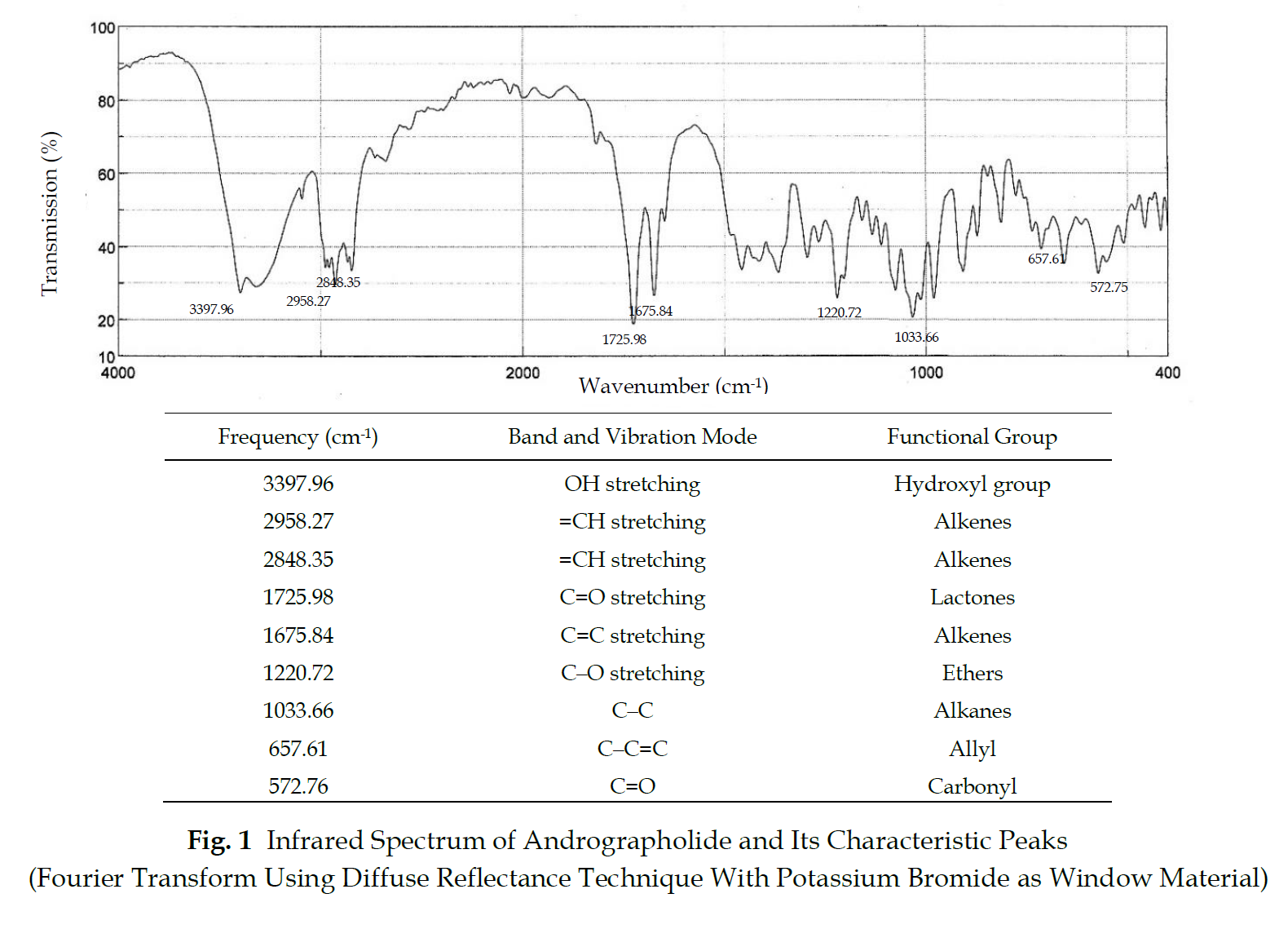
Identification The infrared absorption spectrum corresponds to the spectrum obtained from Andrographolide RS (Fig. 1).
Melting temperature 230° to 231° (Appendix 4.3).
Solubility test A solution of 250 mg in 25 mL of a mixture of chloroform and methanol (1:1) is clear.
Assay Carry out the determination as described in the “Liquid Chromatography” (Appendix 3.5). (Note Filter Standard preparations (a) and (b), Assay preparation and Mobile phases through a membrane having a 0.22-μm porosity or finer before subjecting into the chromatograph.)
Standard stock preparation A solution containing 250 μg per mL of Andrographolide RS in a mixture of equal volumes of methanol and acetonitrile.
Standard preparation (a) Prepare a solution containing 125 μg per mL of andrographolide by quantitatively diluting Standard stock preparation with a 0.050 per cent v/v solution of formic acid.
Standard preparation (b) Weigh accurately a quantity of Powdered Andrographis Extract RS equivalent to about 25 mg of diterpene lactones. Dissolve in 25 mL of a mixture of equal volumes of methanol and acetonitrile. Sonicate or heat gently for 15 to 20 minutes. Dilute further with the same solvent to obtain a solution having a known concentration of about 240 μg per mL of diterpene lactones.
Assay preparation Weigh accurately a suitable quantity of Andrographolide, dissolve in and dilute with a mixture of equal volumes of methanol and acetonitrile to obtain a solution containing 250 μg per mL of andrographolide. Dilute further with a 0.050 per cent v/v solution of formic acid to have a solution of a known concentration of about 125 μg per mL of andrographolide.
Mobile phase:
MOBILE PHASE A A 0.050 per cent v/v solution of formic acid.
MOBILE PHASE B A 0.025 per cent v/v solution of formic acid in acetonitrile for chromatography.
The step gradient of mobile phases is as follows:
Time
(Minutes) |
Mobile Phase A
(Per Cent V/V) |
Mobile Phase B
(Per Cent V/V) |
| 0 |
90 |
10 |
| 4.5 |
55 |
45 |
| 6.25 |
20 |
80 |
| 7.00 |
20 |
80 |
| 8.00 |
90 |
10 |
| 10.00 |
90 |
10 |
Chromatographic system
DETECTOR Ultraviolet light (223 nm)
COLUMN A stainless steel column (10 cm × 2.1 mm), packed with octadecylsilane chemically bonded to porous silica or ceramic microparticles (1.7 μm). (Waters-Acquity® or equivalent is suitable)
COLUMN TEMPERATURE 40°
FLOW RATE 0.5 mL per minute
System suitability
SAMPLE Standard preparation (a) and Standard preparation (b).
SUITABILITY REQUIREMENTS The chromatogram of the standard preparation (b) is concordant with the reference chromatogram (Fig. 2). The approximate relative retention times of andrographolide, neoandrographolide, 14-deoxy-11,12-didehydroandrographolide, and andrograpanin are 1.00, 1.32, 1.45, and 1.75, respectively, with reference to the andrographolide peak.
Column efficiency Not less than 5000 theoretical plates for the andrographolide peak in the chromatogram of the standard preparation (a).
Symmetry factor Not more than 1.5 for the andrographolide peak in the chromatogram of the standard preparation (a).
Resolution Not less than 5 between the neoandrographolide and 14-deoxy-11,12-didehydroandrographolide peaks in the chromatogram of the standard preparation (b).
Relative standard deviation Not more than 2.0 per cent for andrographolide in the chromatogram of the standard preparation (a).
Procedure Separately inject equal volumes (about 2 μL) of Standard preparation (a) and Assay preparation into the chromatograph, record the chromatograms and measure the responses for the major peak.
Calculation Calculate the content of C20H30O5 in the portion of the Andrographolide taken, using the declared content of C20H30O5 in Andrographolide RS.


